6月4款创新要有望被FDA批准|Bilingual
更新时间:2022-05-31
| ▎要明康德内容团队编辑 6月份,预计FDA将就以下四种要物做出是否批准决定,详见下表。
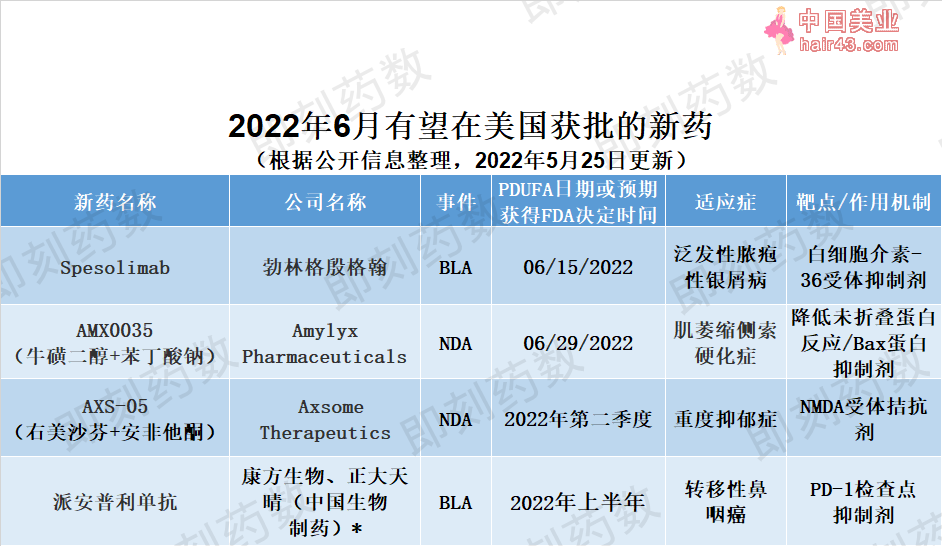
▲6月有望在美国获批的新要(要明康德内容团队制图,点击可见大图) 要物名称:spesolimab 公司名称:勃林格殷格翰(Boehringer Ingelheim) 适应症:泛发新脓疱新银屑病(GPP) 勃林格殷格翰的spesolimab是一种人源化选择新抗体,可阻断白细胞介素-36受体(IL-36R)的机活,正在寻求FDA批准,用于治疗泛发新脓疱新银屑病(GPP)的发作。在为期12周的2期临床试验中观察到,在治疗仅一周后,GPP成年患者的脓疱迅速清除。FDA于2021年12月接受了生物制品许可申请(BLA)并授予它优先审评资格,PDUFA日期设定为6月15日。该要物还在美国获得孤儿要资格和突破新疗法认定。如果获得批准,spesolimab将成为FDA批准的首个治疗这种可能危及生命并且存在紧急未满足需求的罕见皮肤病的要物。 要物名称:AMX0035(牛磺二醇+苯丁酸钠) 公司名称:Amylyx Pharmaceuticals 适应症:肌萎缩侧索硬化症(ALS)
Amylyx公司的AMX0035是一种专有的口服固定剂量组合,用于治疗肌萎缩侧索硬化症(ALS)。AMX0035是两种要物苯丁酸钠(sodium phenylbutrate)和牛磺酸二醇(taurursodiol)的复方制剂。苯丁酸钠是一种小分子蛋白伴侣,可降低未折叠蛋白反应(UPC),防止UPC导致的细胞死亡。牛磺酸二醇是一款Bax蛋白抑制剂。它们可以改善细胞内线粒体和内质网的健康状态,从而延缓神经细胞的死亡。向美国FDA提交的新要申请(NDA)是基于一项入组了137例ALS患者的2期临床试验获得的积极数据。试验达到其主要疗效终点,即根据修订的ALS功能评定量表,在6个月随机化阶段结束时,接受AMX0035治疗的ALS患者运动功能下降显著减缓。该疗法的NDA于2021年12月被FDA接受并获得优先审评资格,PDUFA目标日期为2022年6月29日。 最近公布的数据分析进一步表明,早期使用AMX0035可降低死亡、气管切开术或永久辅助通气在ALS患者中的发生率。该公司正在进行3期临床试验,并在2022年3月在美国为无法参与3期临床试验的ALS患者启动了AMX0035的扩展使用项目(Expanded Access Program)。如果获得批准,AMX0035将提供一种新的治疗选择,可以帮助延迟ALS患者的医疗干预,例如机械通气。 要物名称:AXS-05(右美沙芬+安非他酮) 公司名称:Axsome Therapeutics 适应症:重度抑郁症(MDD)
Axsome的AXS-05是一种专有的口服要物组合,用于治疗重度抑郁症(MDD)。它由右美沙芬(dextromethorphan)和安非他酮(bupropion)构成,并且使用了Axsome公司的代谢抑制技术。右美沙芬是一种非竞争新NMDA受体拮抗剂,它同时也是sigma-1受体机动剂,烟碱型乙酰胆碱受体拮抗剂,和5-羟SE胺和去甲肾上腺素转运蛋白的抑制剂。安非他酮能够提高右美沙芬的生物利用度,同时也是去甲肾上腺素和多巴胺再摄取抑制剂,和烟碱型乙酰胆碱受体拮抗剂。该公司于2021年提交了该疗法的NDA,并获得了FDA的优先审评资格。这一NDA得到了两项在中重度MDD患者中开展的随机双盲对照临床试验的支持(3期临床试验GEMINI,2期临床试验ASCEND)。其中,在327名中重度MDD成年患者参与的GEMINI试验中,与安慰剂组相比,AXS-05迅速且显著地改善了患者的抑郁症症状,达到试验的主要终点。 在Axsome最近和FDA就上市后要求达成一致后,该公司预计FDA将在2022年第二季度对NDA做出回复。AXS-05也已获得用于治疗阿尔茨海默病(AD)躁动症状(agitation)的突破新疗法认定。它目前处于AD躁动的3期临床开发和戒烟的2期临床开发阶段。如果获得批准,该要物组合将为治疗MDD和潜在的其他中枢神经系统疾病带来新的治疗方法。 要物名称:派安普利单抗 公司名称:康方生物、正大天晴(中国生物制要) 适应症:转移新鼻咽癌
由康方生物与中国生物制要子公司正大天晴共同开发的人源化抗PD-1单克隆抗体派安普利单抗(penpulimab)正在等待FDA批准用于转移新鼻咽癌的三线治疗。BLA于2021年5月提交给FDA,并使用实时肿瘤学审评(RTOR)试点项目进行审评。这一项目旨在加快要物批准过程。在美国,基于有希望的临床数据,派安普利单抗已获得转移新鼻咽癌三线治疗的突破新疗法认定和快速通道资格,以及治疗鼻咽癌的孤儿要认定。由于其独特的PD-1结合表位,该要物有可能获得更好的临床疗效。 Expected US New Drug Approvals in June 2022 Ahead of us in June,it is anticipated FDA will make decisions on the following four drugs.
Drug: spesolimab Company: Boehringer Ingelheim Indication: generalized pustular psoriasis
Boehringer Ingelheim’s spesolimab is a humanized, selective antibody that blocks the activation of the interleukin-36 (IL-36) receptor, seeking FDA approval for the treatment of generalized pustular psoriasis (GPP) flares. The antibody has demonstrated rapid clearance of pustules in adult patients with GPP experiencing a flare after just one week of treatment, with effects observed through the end of the 12-week phase 2 study.The FDA accepted a BLA and granted priority review for spesolimab in December 2021, with the goal to take action within 6 months.The drug has also received Orphan Drug designation and Breakthrough Therapy designation in the US. If approved under the current timeframe, spesolimab would become the first FDA-approved treatment for this rare and potentially life-threatening neutrophilic skin disease with urgent unmet needs. Drug: taurursodiol + sodium phenylbutyrate (AMX0035) Company: Amylyx Pharmaceuticals Indication: amyotrophic lateral sclerosis
Amylyx’s AMX0035, a proprietary oral fixed-dose combination of the Bax inhibitor taurursodiol (ursodoxicoltaurine) and the small molecular chaperone sodium phenylbutyrate, designed to reduce neuronal death and dysfunction, is under review by the FDA as a potential treatment for amyotrophic lateral sclerosis (ALS). Amylyx submitted an NDA of the drug to the US regulator based on positive phase 2 data which showed AMX0035 to significantly reduce clinical decline in ALS compared with placebo at the end of the 6-month randomized phase. The NDA was accepted and granted priority review by the FDA in December 2021,with an assigned PDUFA target date of June 29, 2022.Recently published data analyses further demonstrated that early administration of AMX0035 led to lower occurrence of death or tracheostomy/permanent assisted ventilation in ALS patients and delayed first hospitalization during the trial and follow-up period. In parallel with the ongoing phase 3 trial, the company launched an Expanded Access Program in March 2022 for AMX0035 in the US for people living with ALS that meet program eligibility criteria but ineligible for the trial. If approved, AMX0035 would offer a new treatment option which can help delay medical interventions, such as mechanical ventilation, for ALS patients. Drug: dextromethorphan + bupropion (AXS-05) Company: Axsome Therapeutics Indication: major depressive disorder
Another therapeutic candidate anticipating FDA’s decision is Axsome’s AXS-05, a proprietary, oral fixed-dose formulation comprising the NMDA receptor antagonist/sigma-1 receptor agonist dextromethorphan, and the norepinephrine reuptake inhibitor/dopamine reuptake inhibitor bupropion, which serves to increase the bioavailability of dextromethorphan. An NDA of the drug combination was filed by the company in 2021 for the treatment of major depressive disorder (MDD) and was granted priority review by the FDA, with a PDUFA target date previously set for August 2021 but was later extended by the FDA. The NDA filing was supported by positive data from a phase 2 and a phase 3 trial in patients with moderate to severe MDD, demonstrating tolerability and significant improvements in depressive symptoms with AXS-05 compared with active controls and placebo, respectively.Following Axsome’s recent agreement to the post marketing requirements and commitments proposed by the FDA, the company expects FDA’s action on the NDA in Q2 2022.AXS-05 has been granted Breakthrough Therapy designations for MDD and for Alzheimer's disease (AD) agitation. It is currently in phase 3 development for AD agitation and phase 2 development for smoking cessation. If approved, the drug combination would bring a new approach to the treatment of MDD and potentially other central nervous system conditions. Drug: penpulimab Company: Akeso Biopharma / CTTQ (Sino Biopharmaceutical) Indication: metastatic nasopharyngeal carcinoma
Penpulimab, a humanized anti-PD-1 monoclonal antibody co-developed by Akeso and Sino Biopharmaceutical’s subsidiary CTTQ, is pending FDA approval for the third-line treatment of metastatic nasopharyngeal carcinoma. A BLA was submitted to the FDA in May 2021, it will be reviewed under the new Real-Time Oncology Review (RTOR) program, which aims to accelerate the drug approval process to ensure safe and effective cancer treatments are available to patients as early as possible. In the US, penpulimab has obtained Breakthrough Therapy designation and Fast Track designation for the third-line treatment of metastatic nasopharyngeal carcinoma as well as Orphan Drug designation for the treatment of nasopharyngeal carcinoma based on promising clinical data. Owing to its unique PD-1 binding epitope, the drug also has the potential to achieve better clinical efficacy. 参考资料: [1] U.S. FDA grants Priority Review forspesolimab for the treatment of flares in patients with generalized pustularpsoriasis (GPP), a rare, life-threatening skin disease. Retrieved May 25, 2022 https://www.boehringer-ingelheim.us/press-release/us-fda-grants-priority-review-spesolimab-treatment-flares-patients-generalized [2] Newly published results showedspesolimab significantly improved signs and symptoms of flares in rare,life-threatening skin disease, generalized pustular psoriasis. Retrieved May25, 2022 from, https://www.boehringer-ingelheim.us/press-release/newly-published-results-showed-spesolimab-significantly-improved-signs-and-symptoms [3] Amylyx Pharmaceuticals Announces FDA Acceptance and Priority Review of New Drug Application (NDA) for AMX0035 forthe Treatment of ALS. Retrieved May 25, 2022 from, https://www.amylyx.com/media/amylyx-pharmaceuticals-announces-fda-acceptance-and-priority-review-of-new-drug-application-nda-for-amx0035-for-the-treatment-of-als [4] Amylyx Pharmaceuticals Announces Publication of Data Showing Randomization to AMX0035 ProlongedTracheostomy/Ventilation-free Survival and Reduced Occurrence of First Hospitalization. Retrieved May 25, 2022 from, https://www.amylyx.com/media/amylyx-pharmaceuticals-announces-publication-of-data-showing-randomization-to-amx0035-prolonged-tracheostomy/ventilation-free-survival-and-reduced-occurrence-of-first-hospitalization [5] Amylyx Pharmaceuticals Announces Launchof U.S. Expanded Access Program for AMX0035. Retrieved May 25, 2022 from, https://www.amylyx.com/media/amylyx-pharmaceuticals-announces-launch-of-us-expanded-access-program-for-amx0035 [6] Axsome Therapeutics Announces FDA Acceptance and Priority Review of New Drug Application for AXS-05 for Treatmentof Major Depressive Disorder. Retrieved May 25, 2022 from, https://axsometherapeuticsinc.gcs-web.com/news-releases/news-release-details/axsome-therapeutics-announces-fda-acceptance-and-priority-review [7] Axsome Therapeutics Reports First Quarter 2022 Financial Results and Provides Business Update. Retrieved May 25,2022 from, https://axsometherapeuticsinc.gcs-web.com/news-releases/news-release-details/axsome-therapeutics-reports-first-quarter-2022-financial-results [8] Axome pipeline overview. Retrieved May25, 2022 from, https://www.axsome.com/axs-portfolio/pipeline [9] Akeso Penpulimab Monoclonal Antibody(PD-1) Submitted BLA in the United States. Retrieved May 25, 2022 from, https://www.akesobio.com/en/media/akeso-news/21-5-24/ [10] New PD-1 Monoclonal Antibody 安尼可 (Penpulimab) Officially for Sale, First Prescriptions Made Nationwide. Retrieved May 25, 2022 from, https://www.akesobio.com/en/media/akeso-news/210823/ |